Prevention of needle and stick injuries
EU directive on prevention of needle stick injuries.
Health sector employees and employers throughout Europe stand to benefit from Directive 2010/32/EU.
Background
Injuries from medical sharps, including needle sticks (scalpels, needles and cannulas) are one of the largest risks for workers in the hospital environment. Even the tiniest skin lesionLesion (from the Latin "laesio" - injury) is the general medical term for any injuries and pathologic changes in the human body or disorders of bodily functions. can mean contact with a patient’s blood possibly leading to serious infectious diseases such as hepatitis B or C or HIV. Experts assume on average one such injury per employee per year.
A further problem is that the effected employees tend to trivialise the injury. Many treat their own injuries and do not report them. However, for the injured worker, whether doctor, nurse or cleaning staff, it is extremely important that the injury is reported and laboratory tests are carried out straight away.
If an infection is confirmed, immediate measures can be taken. Moreover, reporting the incident also means that it can be recognised as an occupational illness. If no serious infection is found, the result liberates the injured person from uncertainty.
The aim of the directive is to reduce the risk of needle stick injuries for health sector employees throughout Europe by implementing different measures to achieve the safest working environment. Reducing this type of incident also has an economic value as less working hours are lost as a result of injuries and the costs for examinations and treatment are reduced. (Each event is assumed to cost between 487 €and 3.465 €–not including the cost of follow-up treatments).
Effective prevention
Recent studies have shown that the incidents can be reduced considerably by applying certain measures after an in-depth risks evaluation of each area: appropriate education of staff, training on proper handling of instruments, raising risk awareness, improvement of working conditions as well as the usage of safety instruments with integrated protection mechanisms and special containers for sharps disposal.
Guidelines and implementation
For many years, efforts have been directed towards driving prevention in this area forward. However these efforts have not yet resulted satisfactory outcomes. The deadline for the implementation of the EU directive on prevention of sharps injuries ended on May 11th. This means that each EU member state is required to bring into force national legislation or legally binding agreements to implement the directive. The directive specifies minimum requirements and the Member states are free to adopt additional measures to protect workers. The requirements can be summarised in 5 points:
1. working together for better results
Employer and employee representatives shall work together at the appropriate level to eliminate and prevent risks, protect the health and safety of workers and create a safe working environment. Together they are to develop:
- a general prevention strategy
- training programmes
- procedures for health Monitoring
2. Assessment of risks
Risks must be evaluated in all work areas and situations where potential for injuries exists or exposure to blood or other potentially infectious materials is possible.
3. take preventive measures
If the risk evaluation shows increased risk of infection as a result of exposure to patient blood, the following measures are urgently necessary and must be systematically introduced into hospital procedure:
- avoid unnecessary use of sharp/pointed instruments by implementing changed processes
- establish and introduce safe procedures for handling, and disposing of sharp/pointed medical instruments and contaminated waste
- with immediate effect, recapping of used needles with protective caps is forbidden
- provide medical devices with integrated safety and protection mechanisms (e.g. Feather® safety scalpels with blade protection mechanism)
- introduce effective disposal procedures (e.g. robust sharps containers and disposal bins for used needles, cannulas etc.)
- ensure use of personal protective equipment (e.g. gloves, face masks, gowns etc.)
- make appropriate vaccinations available to employees
4. Information and awareness-raising
All employees, and especially all new staff members starting work, shall receive a focused introduction and practical instruction on:
- the correct use of sharp/pointed medical instruments with integrated protective mechanisms
- risks associated with blood or body fluids exposure
- preventative measures including standard precautions, safe systems of work (including the ban on recapping) and, the correct use of sharps bins and disposal procedures
- the importance of immunisation and how to Access immunisation services
- the reporting, response and monitoring procedures and their importance
- measures to be taken immediately in cases of injury
Employers have a duty to provide such training sessions on a regular basis and to release employees so that they can part in them.
5. Reporting must be taken seriously
To protect and support injured workers, accidents and injuries with sharp/pointed devices must be reported to the employer immediately.
Safety devices
According to the guidance of the European Biosafety Network, safety devices meet the following criteria:
- The device must not compromise patient care.
- The device must perform reliably.
- The safety mechanism must be an integral part of the safety device, not a separate accessory.
- The device must be easy to use and must require only minimal changes of technique on the part of the health professional
- The activation of the safety mechanism must be convenient and allow the care-giver to maintain appropriate control over the procedure
- The device must not create other safety hazards or sources of blood exposure
- A single-handed or automatic activation is preferable
- The activation of the safety mechanism must manifest itself by means of an audible, tactile or visual sign to the health professional
- The safety mechanisms should not be easily reversible once activated
Burden of proof
If employees suffer damage as a result of coming into contact with patients’ blood, it is up to the employer to provide appropriate documentation proving compliance with the protective measures identified or explaining according reasons for non-compliance.
Legislation
- Council Directive 2010/32/EU of 10 May 2010 implementing the Framework Agreement on Prevention from Sharp Injuries in the Hospital and Healthcare Sector concluded by HOSPEEM and EPSU, Brussels 10 May 2010
- European Biosafety Network: Implementation Guidance for the EU Framework Agreement, Council Directive and Associated National Legislation
References
- Berger D., Kirchner G., Labenz L.: Bagatellverletzungen und Infektionsrisiko. In: Hofmann, Reschauer, Stößel(Hrsg): Arbeitsmedizin im Gesundheitswesen 2000; 13: 147–154, Edition FFAS, Freiburg/Breisgau.
- Gemeinschaftsinitative „Safety First“: Proaktives Sicherheitsmanagement im Gesundheitsdienst – Prävention von Nadelstichverletzungen.
- Gerberding J.L.: Occupational exposure to HIV in health care settings. New Engl J Med 2003; 348: 826–833. N Engl J Med. 2003 Feb 27;348(9):826-33
- Graf-Deuel E., Germann D., Martens A. et al.: Einschätzung des unfallbedingten Infektionsrisikos durch HBV, HCV und HIV beim Personal des Kantonsspitals St. Gallen unter spezieller Berücksichtigung präventiver Maßnahmen. In: Hofmann, Reschauer, Stößel (Hrsg): Arbeitsmedizin im Gesundheitswesen 2001; 14: 91–99, Edition FFAS, Freiburg/Breisgau.
- Graf-Deuel E.: Auswertung der Stichverletzungen am KSSG Jahre 2000, 2001 und 2002, Personalärztlicher Dienst. St. Gallen 2002
- Kralj N.: Nadelstichverletzungen im Gesundheitsdienst: Vorkommen, Folgen und Vorbeugung. Zahnärztliche Mitteilungen 2002; 19: 34–36.
- N.N.: Needle stick injuries: nurses at risk, Mich Nurse. 2000 Mar;73(3):8-9.
- Wagner-Ferrer D., Hartmann W.: Kastenanalyse einer Nadelstichverletzung, Anästh Intensivmed 2006;47:63-66
- Wittmann A.: Kosten von Nadelstichverletzungen und wirtschaftlicher Nutzen neuer Sicherheitsprodukte. Prakt. Arb. med. 2006;5: 40-41
- Wittmann A., Vrka Z., Neukirch B., Hofmann F.: Gesamtwirtschaftliche Kosten durch Nadelstichverletzungen und möglicher Nutzen durch die Einführung Sicherer Instrumente (V 87), Beitrag auf der 47. Jahrestagung der DGAUM am 24.3.2007 in Mainz, zur Veröffentlichung angenommen
Quicklinks
pfm medical guide to the EU directive on prevention of needlestick injuries
(365 kb, pdf)
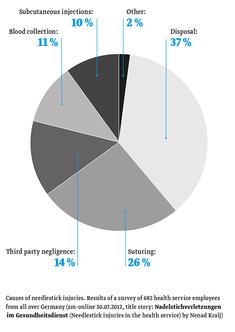
Reasons for needlestick injuries
Contact
Please get in touch if you have any questions about our safety products and their everyday clinical use.
We'll be glad to help.
T +49 2236 9641-221