"PRO-BRA"-Study
National, Multicentre Post-Market Surveillance Study "Patient Reported Outcome" in Breast Reconstruction Following Mastectomy With Titanised Polypropylene Mesh (TiLOOP® Bra)
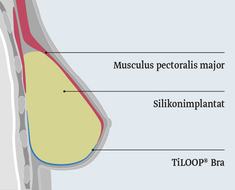
This national, multicentre post-market surveillance study examined the titanised polypropylene mesh implant TiLOOP® Bra which is already on the market. TiLOOP® Bra is intended for the support, reinforcement and bridging of body's own tissue in reconstructive and plastic-aesthetic breast surgery, e.g. after breast cancer.
Method
Between December 2013 and July 2016, 269 patients were enrolled in a prospective, single-arm, multicentre study. The patients underwent a skin-sparing, nipple-sparing or modified radical mastectomy followed by primary or secondary breast reconstruction with the titanised mesh TiLOOP® Bra.
Study objectives
Primary Enpoint
- Assessment of the quality of life after plastic-reconstructive surgery:
Comparison pre- and 12 months postoperative using the Breast-Q questionnaire reconstructive
Secondary Endpoint
- Assessment of the quality of life at 6 and 24 months after breast reconstruction using the Breast-Q questionnaire reconstructive
- Documentation of the complication rate after 6, 12 and 24 months
- Evaluation of the cosmetic outcome after 6, 12 and 24 months
- Documentation of the recoveryConvalescence (from the Latin "reconvalescere" - becoming strong again) means healing or recovery. time in comparison to other breast surgery procedures
Status of the study
Completed
Study centers
- Agaplesion Markus Krankenhaus, Frankfurt am Main
- Charité Campus Mitte und Benjamin Franklin, Berlin
- Helios Kliniken, Berlin
- Klinik für Frauenheilkunde und Geburtshilfe, Lübeck
- Klinikum rechts der Isar, München
- Sankt Gertrauden-Krankenhaus, Berlin
- St. Elisabeth Krankenhaus, Köln
- Vivantes Kliniken am Urban, Berlin
Independent experts to assess the cosmetic outcome:
- Klinik für Frauenheilkunde und Brustzentrum, Universitätsmedizin Greifswald
- Rems-Murr-Klinikum Winnenden
Further study details
Publication
- Patient reported outcome and cosmetic evaluation following implant-based breast-reconstruction with a titanized polypropylene mesh (TiLOOP® Bra): A prospective clinical study in 269 patients - Thill M., Faridi A., Meiré A., et al. European Journal of Surgical Oncology. 2020, Aug;46(8):1484-1490
- Study summary TiLOOP® Bra Studie (PRO-BRA): Patient Reported Outcome and cosmetic evaluation
Subpectoral reconstruction
Post-market clinical follow up study TiLOOP® Bra Pocket
As part of the product portfolio expansion for pre-pectoral breast reconstruction with TiLOOP® Bra Pocket, the PRO-Pocket study was initiated in July 2019. This study evaluates the "Patient Reported Outcome" (PRO) and the cosmetic outcome after implantation of TiLOOP® Bra Pocket.
Surgery workshops
Find out more about oour Surgery workshops.