Titanisation
Titanium: At home in medicine for more than 70 years
In medicine, titanium is here to stay. With its outstanding characteristics, it meets even the highest demands in medicine. Apart from ideal mechanical properties such as light weight and high tensile strength, tissue and bone cells become particularly well incorporated into titanium implants.
The human body integrates titanium implants exceptionally well because of their superior tolerability. Since 1946, titanium has therefore seen applications in many areas of medicine.1
Following is a small list of titanium implant examples:
- Dental implants
- Implants for hip and knee replacement
- Titanium screws and plates stabilizing fractures
- Auditory ossicle implants
- Titanium clips, e.g. in neurosurgery
These are some examples of full-metal titanium implants, i.e. made of pure titanium. Whenever a high degree of flexibility and/or elasticity was required, for a long time this ruled out titanium, since it is basically an inflexible material.
Mesh implant titanisation
In the year 2000 pfmmedical was able to transfer the benefits of titanium onto a flexible and elastic base material, in particular onto a polypropylene mesh.2

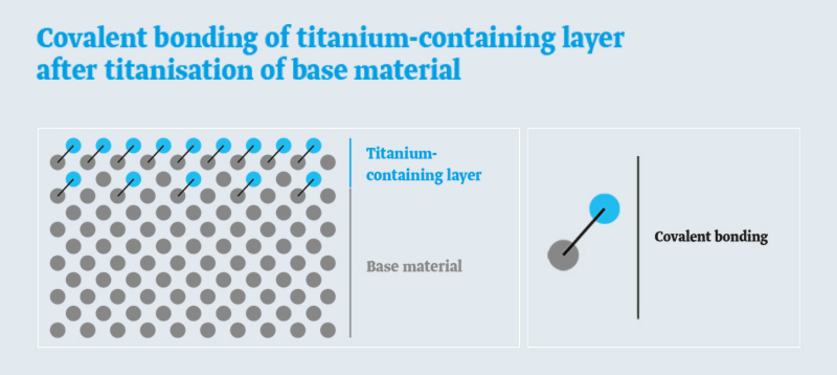
During the unique manufacturing stage of titanisation, the carbon in the titanium-containing layer undergoes covalent bonding with a Type 1a polypropylene mesh. Covalent bonding means that there is a bond at the atomic level.
Titanisation makes surfaces become hydrophilic
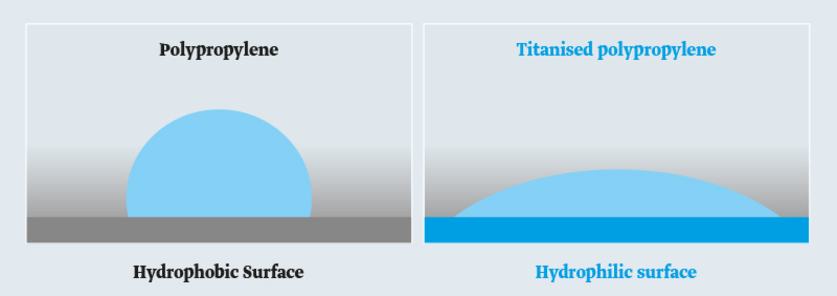
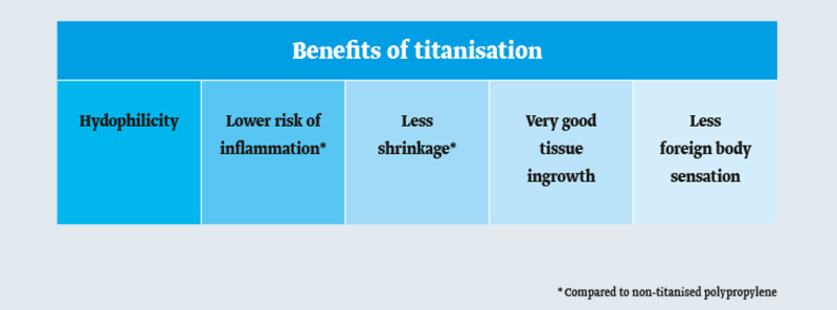
Titanisation results in a hydrophilic (“water-loving”) surface. With titanisation, the originally hydrophobic (“water-repellent”) surface of the polypropylene becomes hydrophilic. A simple test with a water drop will demonstrate this.
On hydrophobic surfaces the water drop will become circumscribed. On titanised and thus hydrophilic surfaces the water drop will disperse.
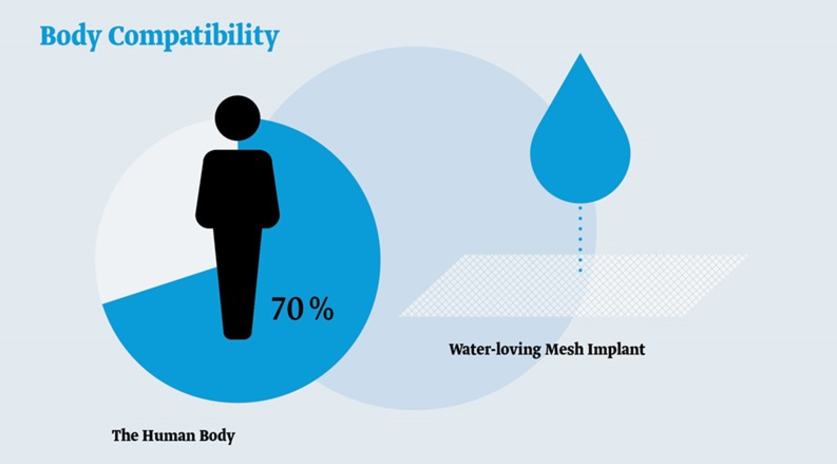
The human body has a water content of approximately 70%. The titanised and therefore water-receptive (hydrophilic) surface of our mesh implants thus gives rise to better tolerability.4,10
Compared with simple hydrophobic polypropylene, the hydrophilic surface of a titanzed mesh implant offers numerous benefits:
The benefits of titanised mesh implants
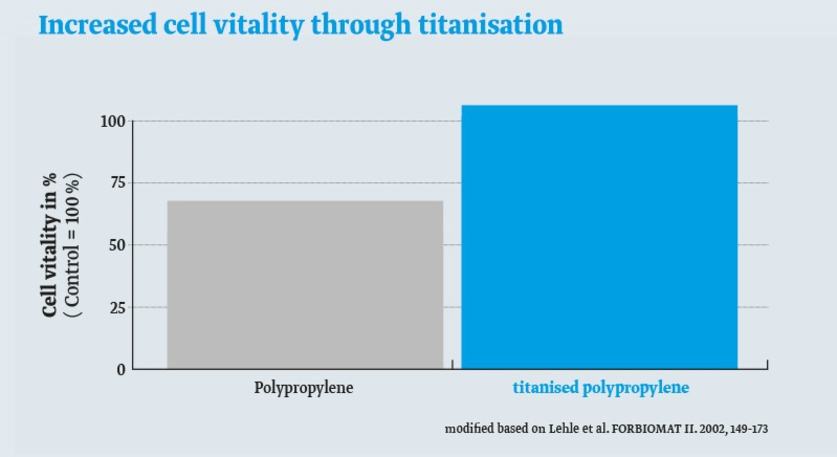
Better cell growth
Unlike on polypropylene surfaces, fibroblasts on titanised surfaces are more energetic and grow better.3
Less risk of inflammation and better compatibility
Every implant triggers an inflammatory reaction. The smaller the reaction, the better the body will tolerate the implant.4
The following figures compare the marker protein levels of inflammatory cells (macrophages and B-lymphocytes) after implantation of various hernia meshes in an animal model. The titanised hernia mesh TiMESH demonstrates the lowest levels of these proteins specific for inflammation.
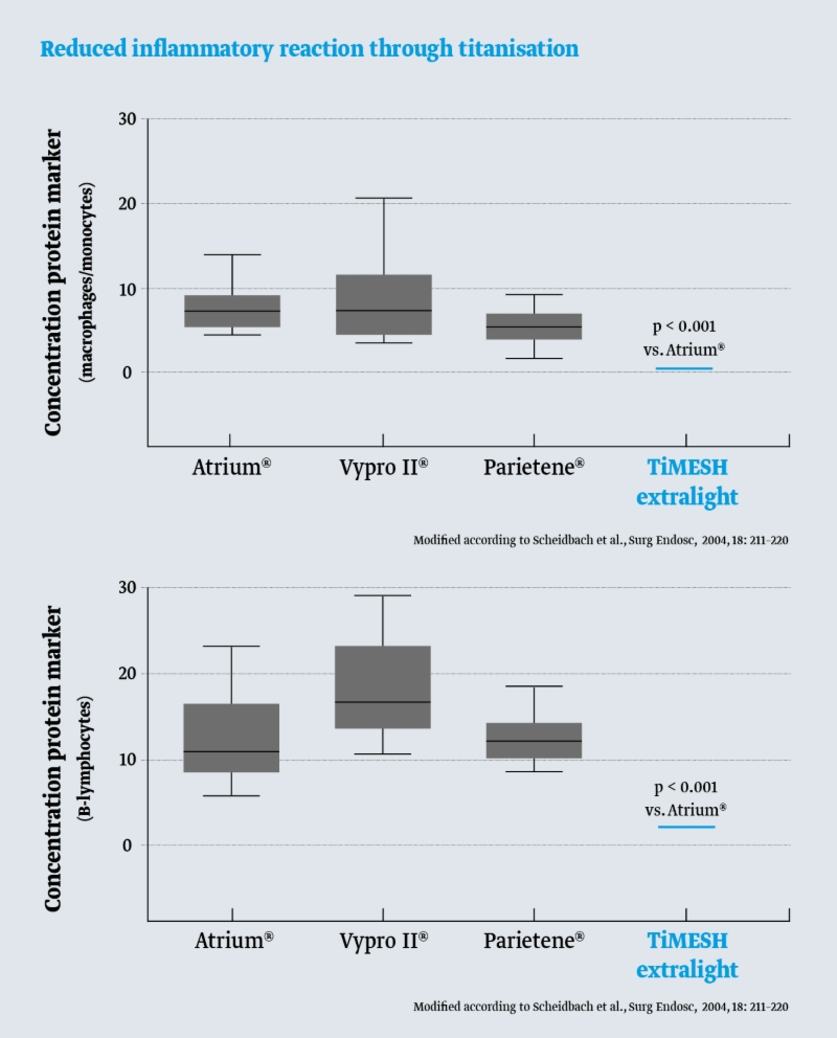
Makrophags/Monozytes and Proteine CD11B, CD1A as markers for B-Lymphozytes.
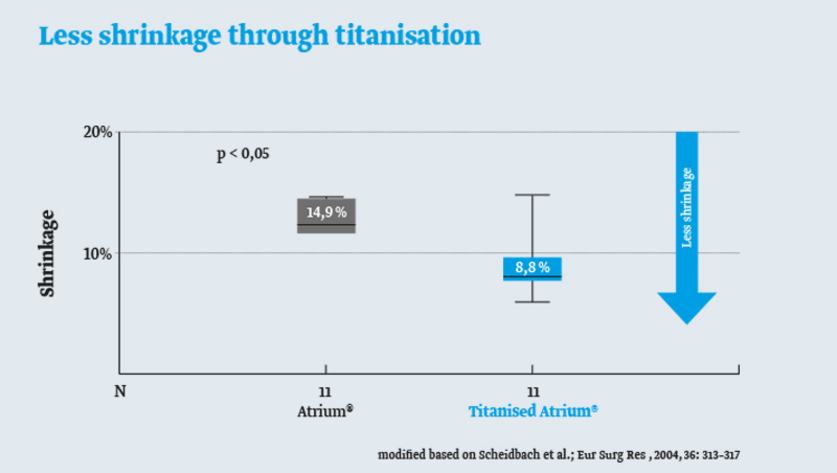
Less scarring and less mesh shrinkage
In general, the stronger the inflammation, the thicker the scar. Since the inflammatory reaction of titanised meshes is smaller than for non-titanised polypropylene meshes, less scarring will take place.5
This has one important consequence: Since the scar tissue encapsulating the mesh will shrink less over time, this also holds true for the mesh.6,7
No significant immune response
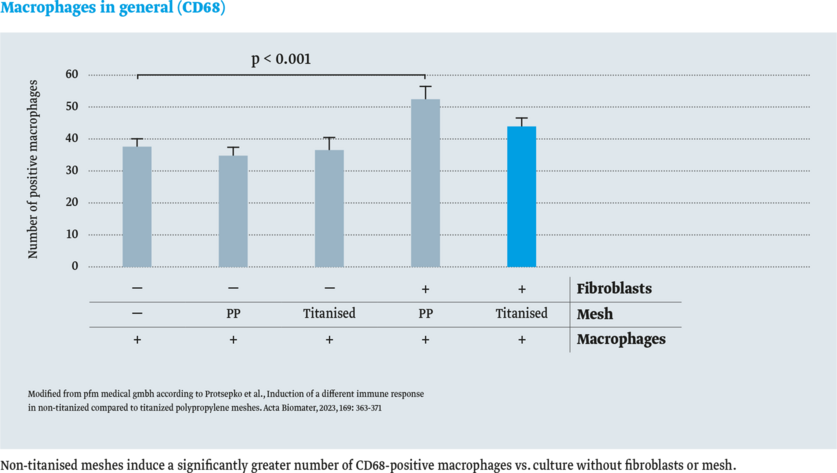
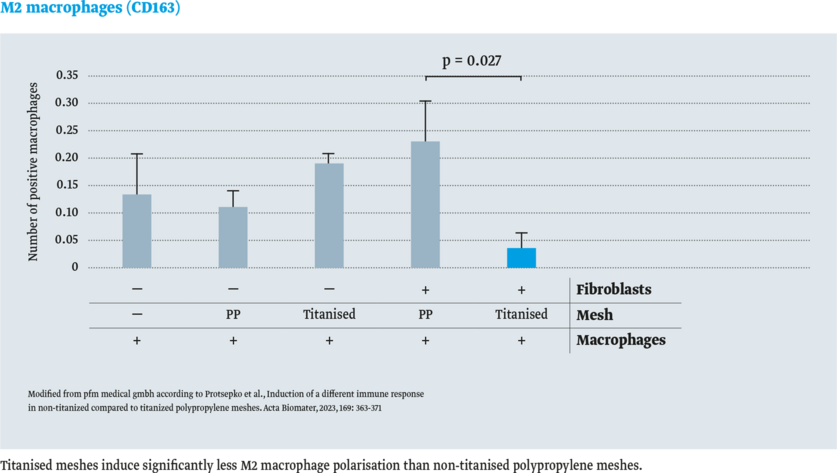
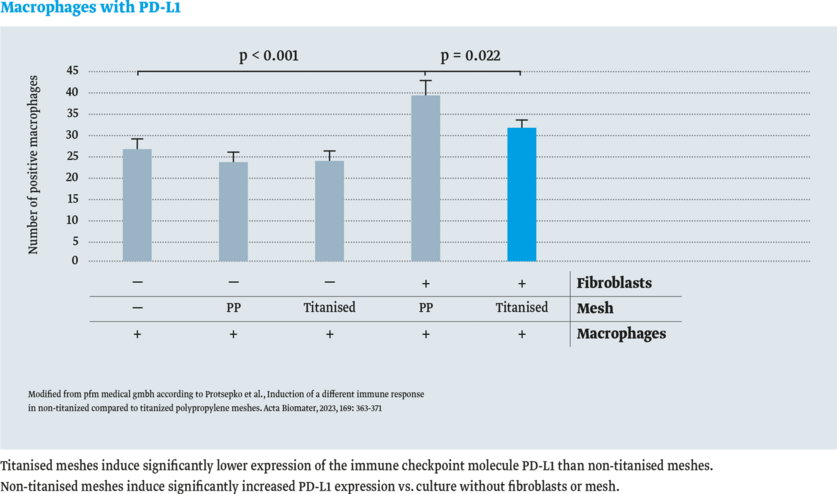
In the study by Protsepko et al. (2023), different immune responses in mesh-materials were investigated.
The immune response in titanised and non-titanised polypropylene (PP) meshes was compared. For this purpose,macrophages were cultured in vitro on the mesh material with and without fibroblasts. Immunohistochemistry was used to detect the following:
- Macrophages in general (CD68)
- M2-polarised macrophages (CD163)
- Immune checkpoint molecule PD-L1 (Programmed Cell Death-Ligand 1)
The result: No significant immune response with titanised meshes in vitro.8
Why is the inflammatory reaction smaller?
Our immune system becomes active whenever it detects foreign bodies. One such example might be proteins displaying unnatural shapes. For example, hydrophilic proteins such as albumins will take on such unnatural shapes whenever they bind to hydrophobic surfaces. This change in shape activates the cells of the immune system, e.g. macrophages and B-lymphocytes. These cells power the inflammatory response terminating in the formation of scar tissue.
Hydrophilic proteins, however, bind to hydrophilic surfaces with very little change in shape. The cells of the immune system are activated far less and the inflammatory response is not as marked.
Since the immune system receives a weaker signal to trigger the inflammatory response, scar tissue formation and mesh shrinkage are less pronounced5,6,7 and the surrounding tissue will be better incorporated into the mesh. The outstanding implant ingrowth characteristic lessens the foreign body sensation.9
Titanisation with its better compabitility is used to improve patient outcome and quality of life: as titanised mesh in hernia surgery, breast surgery and pelvic floor reconstruction.
- Wintermantel, E., S.-W.H., Medizintechnik Life Science Engineering. 5 ed. 2009, Berlin Heidelberg: Springer-Verlag.
- CE-mark for titanised hernia mesh TiMESH: 2002
- Lehle, K., et al., Verbesserung des Langzeitverhaltens von textilen Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte chemische Gasphasenabscheidung (PACVD), in Abschlussbericht: Bayerischer Forschungsverbund Biomaterialien, Bayerischer Forschungsverbund Biomaterialien, Editor. 2003. p. 149-173.
- Scheidbach, H., et al., In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc., 2004. 18(2): p. 211-220.
- Scheidbach, H., et al., Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. An animal experimental model. Eur Surg.Res., 2004. 36(5): p. 313-317.
- Zhu, L.M., et al., Mesh implants: An overview of crucial mesh parameters. World J Gastrointest Surg, 2015. 7(10): p. 226-36.
- Wood, A.J., et al., Materials characterization and histological analysis of explanted polypropylene, PTFE, and PET hernia meshes from an individual patient. J Mater Sci Mater Med, 2013. 24(4): p. 1113-22.
- Protsepko, O., Voisard, P., Kuhn, C., Maccagno, A., Dannecker, C., Jeschke, U., Pauli, F., & Garrido, F., Induction of a different immune response in non-titanized compared to titanized polypropylene meshes. Acta Biomater, 2023, 169: 363-371
- The SAGES Manual of Hernia Repair. 1 ed. 2013, New York: Springer-Verlag
- Horstmann R., et al., Impact of polypropylene amount on functional outcome and quality of life after inguinal hernia repair by the TAPP procedure using pure, mixed, and titanium-coated meshes. World J Surg., 2006. 30(9): p. 1742-1749