TiLOOP® PRO PLUS T
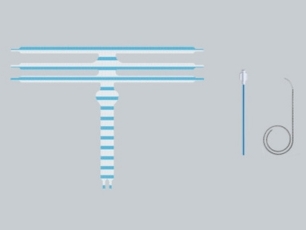
Benefits
Unique PLUS System
With the PLUS-System, which consists of a catheter and loops, the mesh arms can be positioned atraumatically and adjusted bi-directionally: best prerequisites for tension and fold-free insertion of the mesh.
Reduced Risk of Inflammation
Compared to simple polypropylene, the hydrophilic, titanised surface represents a lower risk of inflammation — and therefore less scar formation.1
By urogynaecologists, for urogynaecologists
All TiLOOP® mesh implants were developed together with urogynaecologists and are 100% Made in Germany.
[1] Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
Product Details
- Monofilament fabric (knitted monofilament fibres)
- Laser cut atraumatic edges (rounded)
- Non-absorbable
- EO-sterilised (ethylene oxideEthylene oxide is a gas used as disinfectant for organic insulation (wool, plant fibres), textile fibres, and medical devices and products.), pyrogen free
- Instruments to be ordered separately: TiLOOP® Instruments PLUS (REF 6000964)
Technical Data
- Content: 1 Mesh, 8 Catheters, 8 Snares
- Material: Titanised type 1a polypropylene mesh
- Pore size: 3 mm
- Product weight: 24 g/m²
Knowledge
Highest demands on the material
In addition to the skills of the surgeon, the quality of the mesh material determines the quality of a lasting and anatomically stable descensus repair. TiLOOP® mesh implants are made of Type 1a polypropylene mesh (macroporous & monofilament)1 with a titanised, hydrophilic surface. Compared to simple polypropylene, this offers a number of advantages, which are already known in the use of titanised mesh implants for hernia surgery, such as:
- better cell growth2
- lower risk of inflammation3
- less scarring3
- less shrinkage of the mesh4
[1] Klinge et al. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia (2012) 16: 251–258
[2] Lehle K. Lohn S., Verbesserung des Langzeitverhaltens von Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte Gasphasenabscheidung (PACVD), Abschlussbericht Forschungsverbund “Biomaterialien (FORBIOMAT II)”, 149–173, 2002
[3] Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
[4] Scheidbach et al. Influence of Titanium Coating on the Biocompatibility of a Heavyweight Polypropylene Mesh. Eur Surg Res 2004; 36: 313–317
Manufacturer
- pfm medical titanium gmbh, Südwestpark 42, 90449 Nuremberg, Germany
Ordering Information
| Article number | Marking | Delivery unit (VPE) |
|---|---|---|
| 6001342 | TiLOOP PRO PLUS T | 1 pcs |
Downloads
- Benefits of Titanisation | Brochure (PB4104EN)
- Mesh Implants Pelvic Floor Surgery | Product Brochure (PB4002EN)
Instructions for use can be found in our Download Center.
Additional information

Please note:
Not all products are available in every country.
Please refer to the respective country websites:
For all other countries please use the contact form to enquire about availability for your country.
