TiSURE®
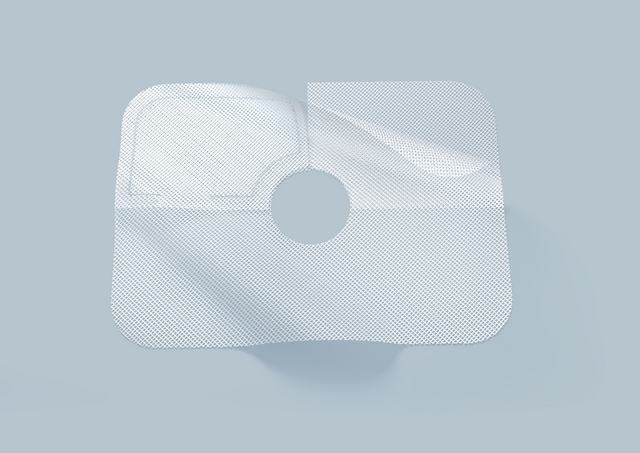
Our complete portfolio of titanised hernia meshes covers all indications. TiSURE® provides excellent patient outcomes thanks to their hydrophilic and therefore very biocompatible properties. The multi-layered mesh TiSURE® serves to reinforce the diaphragm in the event of existing defects at the opening through which the oesophagus passes. The pore size is 1 mm.
Benefits
Body compatibility
Titanium’s body compatibility properties transferred to a hernia mesh
Quality of life
Improved quality of life for patients1
Handling
Easier handling and excellent visibility
Excellent mesh adaptation
The opening can be made larger or smaller, allowing excellent adaptation of the mesh to the diameter of the oesophagus.
Unique design with 360° coverage
Since the mesh is multi-layered, 360° coverage is achieved with adequate mesh overlap, even when the mesh opening is enlarged.
Less mesh shrinkage
The inflammatory response is minimised through titanisation. Consequently there is less scar formation, and therefore less mesh shrinkage.2,3,4 This is particularly important given the area of application of TiSURE®.
No effect on diagnostics
Imaging diagnostics are not affected
[1] Horstmann R., Hellwig M., Classen C., Röttgermann S., Palmes D., Impact of polypropylene amount on functional outcome and quality of life after inguinal hernia repair by the TAPP procedure using pure, mixed, and titanium-coated meshes. World J Surg, 2006, 30(9): 1742-1749
[2] Scheidbach H., Tannapfel A., Schmidt U., Lippert H., Köckerling F., Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. An animal experimental model. Eur Surg Res, 2004, 36(5): 313-317
[3] Zhu L. M., Schuster P., Klinge U., Mesh implants: An overview of crucial mesh parameters. World J Gastrointest Surg, 2015, 7(10): 226-236
[4] Wood A. J., Cozad M. J., Grant D.A., Ostdiek A. M., Bachman S. L., Grant S.A., Materials characterization and histological analysis of explanted polypropylene, PTFE, and PET hernia meshes from an individual patient. J Mater Sci Mater Med, 2013, 24(4): 1113-1122
Product Details
- Monofilament fabric
- Laser cut atraumatic edges
- Non-absorbable
- EO-sterilised (ethylene oxideEthylene oxide is a gas used as disinfectant for organic insulation (wool, plant fibres), textile fibres, and medical devices and products.), pyrogen-free
Technical Data
- Material: Titanised type 1a polypropylene mesh
- Pore size: 1 mm
- Product weight: 35 g/m²
- Size: 7 cm x 10 cm
Knowledge
Highest demands on the material
In addition to the skills of the surgeon, the quality of the mesh material determines the quality of a lasting and anatomically stable hernia repair. TiMESH mesh implants are made of Type 1a polypropylene mesh (macroporous & monofilament)1 with a titanised, hydrophilic surface. Compared to simple polypropylene, this offers a number of advantages, which are already known in the use of titanised mesh implants for hernia surgery, such as:
- better cell growth2
- lower risk of inflammation3
- less scarring3
- less shrinkage of the mesh4
[1] Klinge et al. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia (2012) 16: 251–258
[2] Lehle K. Lohn S., Verbesserung des Langzeitverhaltens von Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte Gasphasenabscheidung (PACVD), Abschlussbericht Forschungsverbund “Biomaterialien (FORBIOMAT II)”, 149–173, 2002
[3] Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
[4] Scheidbach et al. Influence of Titanium Coating on the Biocompatibility of a Heavyweight Polypropylene Mesh. Eur Surg Res 2004; 36: 313–317
Application area
- Hiatal hernias
Manufacturer
- pfm medical titanium gmbh, Südwestpark 42, 90449 Nuremberg, Germany
Ordering Information
| Article number | Delivery unit (VPE) |
|---|---|
| 6000438 | 3 pcs |
Downloads
Instructions for use can be found in our Download Center.
Additional information

Please note:
Not all products are available in every country.
Please refer to the respective country websites:
For all other countries please use the contact form to enquire about availability for your country.