TiLOOP® Bra Pocket


Benefits
Complete portfolio
The TiLOOP® Bra product family covers all indications for breast surgery with tissue reinforcing material.
- TiLOOP® Bra Pocket: Pre-pectoral reconstruction/augmentation
- TiLOOP® Bra: Sub-pectoral reconstruction/augmentation
Optimal capsule quality
Compared to simple polypropylene, the hydrophilic and titanised surface carries a reduced risk of inflammation1 and thus a reduced tendency towards the formation of connective tissue-like scars and shrinkage: combined with minimal weight and ides the ideal conditions for a permanent, stable result as well as both desirable tissue ingrowth and a vascularied, flexible, and therefore optimum capsule quality.
Muscle-preserving, pre-pectoral
The pre-pectoral placement of the implant eliminates the need to detach the muscle from the chest wall and therefore less postoperative pain. The result is a shorter recoveryConvalescence (from the Latin "reconvalescere" - becoming strong again) means healing or recovery. time and the preservation of muscle function. Your patients are less affected in their daily lives.
Excellent aesthetic results
The pre-pectoral placement enables the breast implant to assume the physiological position of the subcutaneous breast tissue, resulting in excellent aesthetics and a natural-looking ptosis.2,3,4
Excellent quality of life
The pre-pectoral reconstruction and the associated benefits improve the patients’ quality of life.3,4
Shorter surgery
TiLOOP® Bra Pocket is a ready-to-use implant. No lengthy fitting procedure, e.g., via intraoperative sutures or hydration, is required. The pre-pectoral reconstruction takes less time than the sub-pectoral reconstruction, since there is no need to prepare the pectoralis major. The patient is therefore anaesthetised for a shorter period.
Protected implant
TiLOOP® Bra Pocket is an implant pocket, which fixes the freely selectable breast implant on the muscle and thus prevents dislocation or twisting. Studies have provided evidence of a low capsule contracture rate, while maintaining an excellent capsule quality.3,4,5
Stretch-optimised implant
The stretch properties of TiLOOP® Bra Pocket have been developed to meet the physiological demands of natural shoulder movements and ptosis.
[1] Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
[2] Casella et al. TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg (2014) 37 (11): 599-604
[3] Bernini et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open (2016) 3 (12):e574
[4] Casella et al. Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction: Long-term Results. Plast Reconstr Surg Glob Open (2016) 3 (12):e577
[5] Gentile P., et al., Titanium-coated polypropylene mesh as innovative bioactive material in conservatives mastectomies and pre-pectoral breast reconstruction. Bioactive Materials, 2021. 6(12): 4640-4653
Product Details
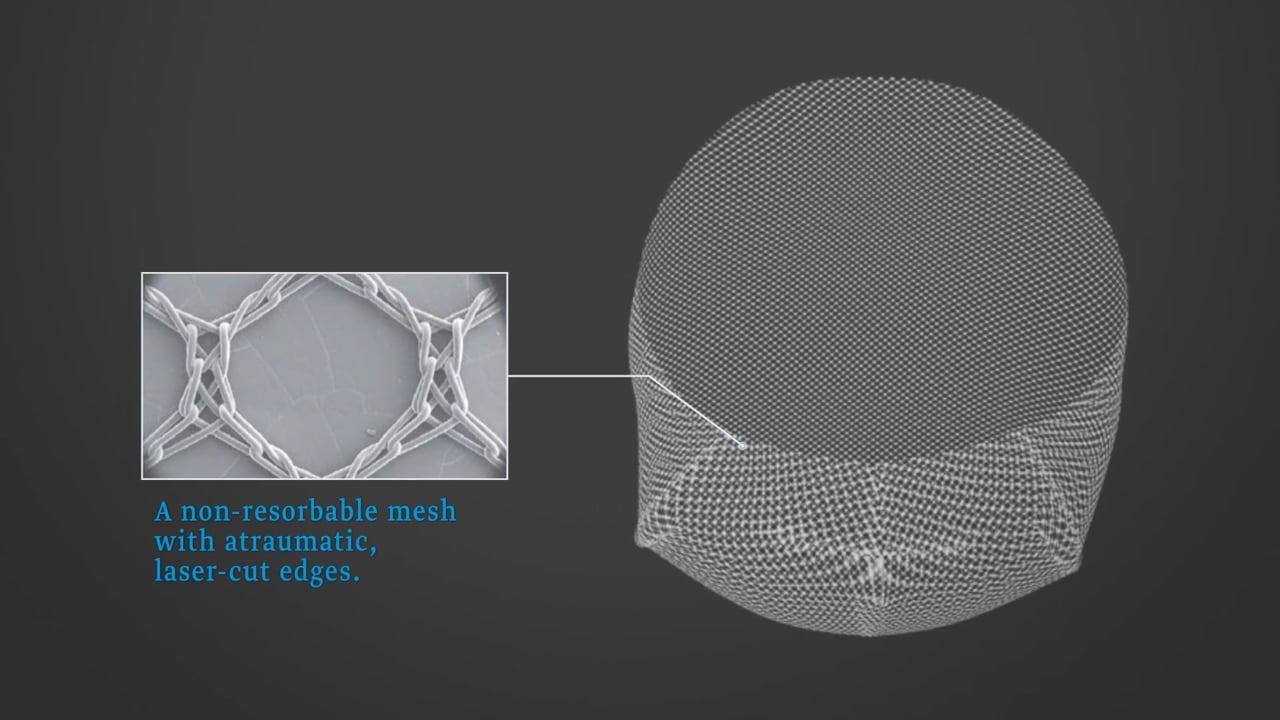
- Monofilament fabric
- Non-resorbable
- Atraumatic, laser-cut edges
- EO-sterilised (ethylene oxideEthylene oxide is a gas used as disinfectant for organic insulation (wool, plant fibres), textile fibres, and medical devices and products.), pyrogen free
Technical Data
- Material: Titanised type 1a polypropylene mesh
- Pore size: 1 mm
- Product weight: 16 g/m²
Knowledge
Orientation Assistance for the selection of the correct mesh size
Small
- Width of implant: < 11 cm
- Projection height of the implant: < 4,5 cm
- Volume of implant: < 270 ml
Medium
- Width of implant: < 13 cm
- Projection height of the implant: < 5,5 cm
- Volume of implant: < 420 ml
Large
- Width of implant: < 15 cm
- Projection height of the implant: < 6 cm
- Volume of implant: < 550 ml
Recommended Implantation Procedure
TiLOOP® Bra Pocket is either fixed on the fascia of the pectoralis major, or directly on the pectoralis major. The implant front, facing the skin, should be completely covered with mesh material. TiLOOP® Bra Pocket undergoes pre-pectoral fixation with cranial, medial and lateral attachment, in order to prevent dislocation of the mesh and implant.
Selection of material
One of the determining factors for successful breast surgery in the long term, is the correct decision for or against the use of tissue reinforcing material (synthetic mesh or ADM).
TiLOOP® Bra mesh implants* are made of Type 1a polypropylene mesh (macroporous, light & monofilament) with a titanised, hydrophilic surface. Compared to simple polypropylene, this offers a number of advantages:
- lower risk of inflammation1,2,3
- better cell growth4
- less scarring5
- less shrinkage of the mesh1
- less postoperative pain6
- High patient satisfaction and improved quality of life2,3
*TiLOOP® Bra mesh implants are not a tissue replacement.
[1] Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
[2] Gentile P., et al., Titanium-coated polypropylene mesh as innovative bioactive material in conservatives mastectomies and pre-pectoral breast reconstruction. Bioactive Materials, 2021. 6(12): 4640-4653
[3] Casella D., et al., Evaluation of prepectoral implant placement and complete coverage with TiLOOP® Bra mesh for breast reconstruction: a prospective study on long-term and patient reported BREAST-Q outcomes, 2019. Plast. Rec. Surg., 143(1):1e-9e
[4] Lehle K., Lohn S. Verbesserung des Langzeitverhaltens von Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte Gasphasenabscheidung (PACVD), Abschlussbericht Forschungsverbund “Biomaterialien (FORBIOMAT II)”, 149–173, 2002
[5] Scheidbach et al. Influence of Titanium Coating on the Biocompatibility of a Heavyweight Polypropylene Mesh. Eur Surg Res (2004) 36: 313–317
[6] Sigalove S., et al., Prepectoral Implant-Based Breast Reconstruction: Rationale, Indications, and Preliminary Results, 2017. Plast. Rec. Surg, 139(2):287-294.
Application area
- Supporting, strengthening and bridging of body’s own tissue structures, as part of reconstructive and plastic-aesthetic breast surgery.
- Reconstructive breast surgery: implant-based reconstruction (also with expander), e.g., after a skin-sparing or nipple-sparing mastectomy.
- Plastic-aesthetic breast surgery: primary or corrective augmentations
Manufacturer
- pfm medical titanium gmbh, Südwestpark 42, 90449 Nuremberg, Germany
Ordering Information
| Article number | Version | Delivery unit (VPE) |
|---|---|---|
| 6001383 | small | 1 pcs |
| 6001385 | medium | 1 pcs |
| 6001387 | large | 1 pcs |
Downloads
- Mesh Implants Breast Surgery | Product Brochure (WPB4300EN)
- Pre-pektoral Reconstruction with TiLOOP Bra Pocket (Lo Torto et al, 2020) | Study Summary (PB4326EN)
- Absorbable Material in Breast Reconstruction | Abstract (PB4324EN)
- Titanised Meshes Breast Surgery | Scientific Publications (PB4319EN)
- Benefits of Titanisation | Brochure (PB4104EN)
Instructions for use can be found in our Download Center.

Please note:
Not all products are available in every country.
Please refer to the respective country websites:
For all other countries please use the contact form to enquire about availability for your country.